1a) i) Define Simple harmonic motion.
ii) Sketch a displacement – time graph for a body performing simple harmonic motion.
b) A uniform cylindrical rod of length 16cm and density 920kgm-3 floats vertically in a liquid of density 1000 kgm-3. The rod is depressed through a distance of 7mm and then released.
- Show that the rod performs simple harmonic motion.
- Find the frequency of the resultant oscillations.
- Find the velocity of the rod when it is at a distance of 5mm above the equilibrium position.
c) What is meant by potential energy?
d) Describe the energy changes which occur when a
- ball is thrown upwards in air.
- loud speaker is vibrating.
2 a) i) Define elastic deformation and plastic deformation
ii) Explain what is meant by work hardening.
b) i) Sketch using the same axes, stress-strain curves for a ductile material and for rubber.
ii) Explain the features of the curve for rubber.
c) A capillary tube is held in a vertical position with one end dipping in a liquid of surface tension γ and density ρ. If the liquid rises to a height, h, derive an expression for h in terms of γ, ρ and radius r of the tube assuming the angle of contact is zero.
d) A mercury drop of radius 2.0 mm falls vertically and on hitting the ground, it splits into two drops each of radius 0.5mm. Calculate the change in surface energy given that surface tension of mercury is 0.52Nm-1.
e) State the effect temperature on surface tension of a liquid.
3.a) State Kepler’s laws of planetary motion.
b) Use Newton’s law of gravitation to derive the dimension of the universal gravitational constant.
c) A satellite is revolving at a height h above the surface of the earth with a period, T.
i) Show that the acceleration due to gravity g on the earth’s surface is given by
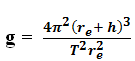 where re is the radius of the earth.
where re is the radius of the earth.
ii) What is meant by parking orbit?
d) A satellite revolves in a circular orbit at a height of 600km above the earth’s surface. Calculate the
- speed of the satellite.
- periodic time of the satellite.
4 a) i) State Newton’s laws of motion.
ii) A molecule of gas contained in a cube of side l strikes the wall of the cube repeatedly with a velocity u.4
b) i) Define linear momentum and state the law of conservation of linear momentum.
ii) A body of mass m1 moving with a velocity u, collides with another body of mass m2 at rest. If they stick together after collusion, find the common velocity with which they will move.
c) A bullet of mass 10g is fired horizontally with a velocity of 300 ms-1 into a block of wood of mass 290g which rests on a rough horizontal floor. After impact, the block and bullet move together and come to rest when the block has travelled a distance of 15m. Calculate the coefficient of sliding friction between the block and the floor.
5 a) i) State the thermometric property used in the constant – volume gas thermometer.
ii) Give two characteristics of a good thermometric property.
b) i) Describe the steps taken to set up a Celcius scale of temperature for a mercury – in – glass thermometer.
ii) State four disadvantages of a mercury-in-glass thermometer.
c) Describe with the aid of a labeled diagram the operation of an optical pyrometer.
d) When oxygen is withdrawn from a tank of volume 50l, the reading of a pressure gauge attached to the tank drops from 21.4 × 105 Pa to 7.8 × 105 Pa. If the temperature of gas remaining in the tank falls from 300C to 100C, calculate the mass of oxygen withdrawn.
6. a) i) Define thermal conductivity.
ii) Explain the mechanism of heat transfer by convection.
b) i) State Newton’s law of cooling.
ii) Describe briefly an experiment to verify Newton’s law of cooling.
c) A wall is constructed using two types of bricks. The temperatures of the inner and outer surfaces of the wall are 290C and 210C respectively. The value of the thermal conductivity for the inner brick is 0.4Wm-1K-1 and that of the outer brick is 0.8Wm-1K-1.
i) Explain why in steady state the rate of thermal energy transfer is the same in both layers.
ii) If each layer is 12.0cm thick, find the temperature at the interface between the layers.
d) Explain the greenhouse effect and how it leads to rise of the earth temperatures.
7 a) i) What is meant by boiling point?
ii) Explain why boiling point of a liquid increases with increase in the external pressure.
b) i) Explain how the pressure of a fixed mass of a gas can be increased at
- constant temperature
- constant volume.
c) i) Sketch a pressure versus volume curve for a real gas undergoing compression.
ii) Explain the main features of the curve in (c) (i) above.
d) The cylinder of an exhaust pump has a volume of 25cm3. If it is connected through a valve to a flask of volume 225cm3 containing air at a pressure of 75cmHg, calculate the pressure of the air in the flask after two strokes of the pump, assuming that the temperature of the air remains constant.
8. a) What is meant by the following:
i) Radioactivity.
ii) Isotopes?
b) i) Define mass defect.
ii) State the condition for a heavy nucleus of an atom to be unstable.
iii) Explain your answer in (b) (ii).
c) A sample of 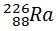 emits both α- particles, each of energy 4.60 MeV and γ- rays, find the frequency of the γ- rays emitted.
emits both α- particles, each of energy 4.60 MeV and γ- rays, find the frequency of the γ- rays emitted.
d) i) Sketch a graph showing the variation of binding energy per nucleon with mass number, clearly showing the fusion and fission regions.
ii) Use the sketch in (d) (i) to explain how energy is released in each of the processes of fusion and fission.
e) State two
i) applications of radioisotopes
ii) health hazards of radioisotopes.
9. a) What are X-rays?
b) i) With the aid of a diagram explain how X – rays are produced in an X-ray tube.
ii) State the energy changes that take place in the production of X-rays in an X-ray tube.
c) In an X-ray tube, the electrons strike the target with a velocity of 3.75× 107 ms-1 after travelling a distance of 5.0cm from the cathode. If a current of 10mA flows through the tube, find the
i) tube voltage
ii) number of electrons striking the target per second.
iii) number of electrons within a space of 1cm length between the anode and the cathode.
d) Briefly explain one medical application of X-rays.
10. a) State Bohr’s postulates of the atom.
b) Explain the occurrence of the emission and absorption line spectra.
c) Explain the occurrence of the emission and absorption line spectra.
d) A beam of alpha particles of energy 3.5MeV is incident normal to a gold foil.
i) Calculate the least distance of approach to the nucleus of the gold atom given its atomic number is 79.
ii) State the significance of the value of the least distance of approach.
END
