Revision Questions in SECTION A
1. Complete the equation below and write the mechanism for the reaction.
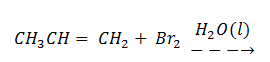
2. a) Write
i) equation for the ionization of benzoic acid in water.
ii) the expression for the ionization constant, Ka of benzoic acid.
b) Calculate the pH of a 0.1M solution of benzoic acid.
(Ka = 6.3 x 10-5 mol dm-3).
3. The first three successive ionization energies of element Y are 549, 1064 and 4225 kJmol-1.
a) Explain the trend in the variation of the ionization energies of Y.
b) State the group in the Periodic Table to which Y belongs.
4. Complete the following equations for nuclear reactions
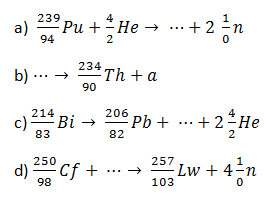
5. When compound W was steam distilled at 860C and 760mmHg pressure, the distillate contained 85% of water by mass. Calculate the relative molecular mass of W.
(The vapour pressure of water at 860C is 740 mmHg)
6 a) 10cm3 of a hydrocaborn P, (CxHy) was exploded in 90cm3 of oxygen. On cooling to room temperature, the residual gases occupied 70cm3. When the residual gases were passed through potassium hydroxide solution, the volume reduced to 40cm3.
i) Write equation for the reaction between P and oxygen.
ii) Determine the molecular formula of P.
b) Write equations to show how P can be prepared from an alcohol.
7. a) State two reasons why fluorine is more reactive than the other elements in group VII in the Periodic Table.
b) Write equation for the reaction between fluorine and
i) water
ii) cold dilute sodium hydroxide solution
iii) hot concentrated sodium hydroxide solution.
8. An organic compound Q has the structure
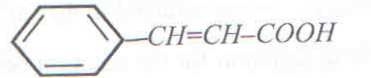
a) Write equation for the reaction that would take place if Q was
i) heated together with methanol in the presence of concentrated sulphuric acid.
ii) treated with aqueous solution of alkaline potassium manganate (VII)
iii) treated with hydrogen bromide.
b) Write a mechanism for the reaction in (a) (iii).
9. a) 2.0g of phosphorous raises the boiling point of 37.4g of carbon disulphide by 1.0030C, whereas 4.65g of sulphur raises the boiling of 100g of carbon disulphide by 0.420C. Calculate the
i) boiling point constant of carbon disulphide. (Molar mass of sulphur is 256)
ii) molar mass of phosphorous in carbon disulphide.
b) Determine the molecular formula of phosphorous.
SECTION B
10. Explain what is meant by molar conductivity.
b) Molar conductivities at infinite dilution, for some compounds at 250C are given in the table below.
|
Compound |
(Ω-1cm2 mol-1) |
|
Na2C2O4 |
248 |
|
H2SO4 |
860 |
|
Na2SO4 |
260 |
|
(NH4)2SO4 |
308 |
Calculate the molar conductivity at infinite dilution for
i) H2C2O4.
ii) (NH4)2C2O4
c) Explain your answers in (b).
d) State two applications of conductivity measurements.
11.a) i) State what is meant by the term thermosetting plastics.
ii) Name one thermosetting plastic.
b) The structural formulae of some polymers are shown in the table below. For each polymer, write the name(s) of monomer(s)
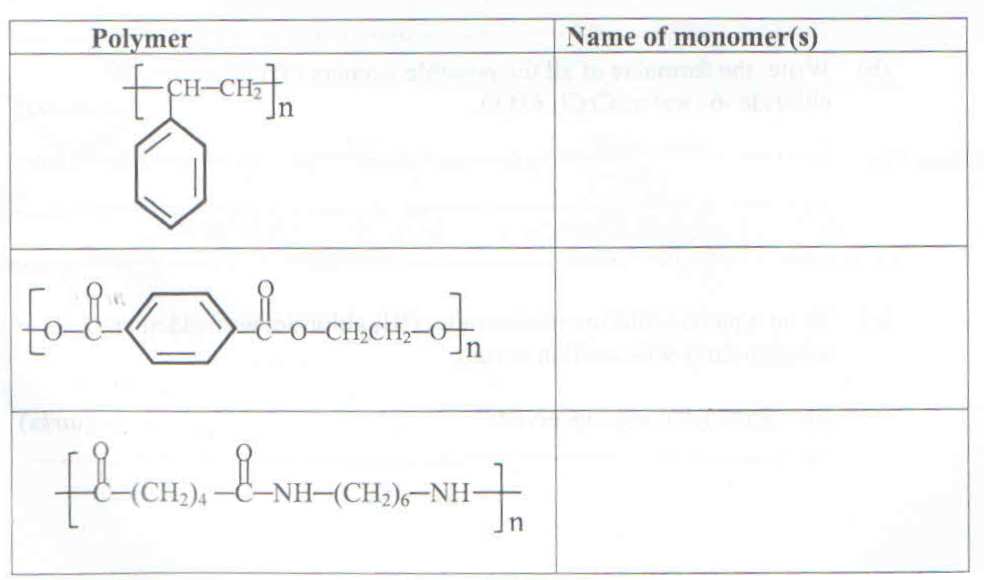
c) The structural formula of polymer, Z is
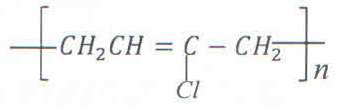
9.89 x 10-2 moles of Z was formed when 350g of the monomer was polymerized. Calculate the
i) value of n.
(H =1; C = 12; Cl= 35.5)
ii) molar mass of the polymer.
12.a) i) Write the electronic configuration of chromium atom,
ii) State why chromium is classified as a transition element.
b) Write the formulae of all the possible isomers of chromium(III) chloride -6 – water, CrCl3.6H2O.
c) To an aqueous solution of chromium (III) chloride, was added ammonia solution drop-wise until in excess.
i) State what was observed.
ii) Write equations for the reactions that took place.
13. Name a reagent that can be used to distinguish between the following pairs of ions. In each case, state what would be observed if each ion is separately treated with the reagent you have named.
a) Ba2+ (aq) and Ca2+ (aq).
Reagent
Observation
b) Br-(aq) and Cl- (aq)
Reagent
Observation
c) CH3CO2-(aq) and C2O42- (aq).
Reagent
Observation
14. Methane reacts with steam according to the following equation.

When 0.625 moles of methane and 3.0 moles of water were heated in a 4dm3 vessel, 2.0 moles of hydrogen was found to be present at equilibrium.
a) i) Write an expression for the equilibrium constant, Kc.
ii) Calculate the value of Kc.
b) The enthalpies of formation of methane, water and carbon dioxide are -76, -242 and -394kJmol-1 respectively.
i) Calculate the enthalpy change for the forward reaction.
ii) State the effect of increasing temperature on the value of Kcand give a reason for your answer.
15. State what would be observed and write equation for the reaction that would take place when
a) hydrogen sulphide gas is bubbled through an acidified solution of potassium dichromate (VI).
Observation
Equation
b) a mixture of proposal and Fehling’s solution is heated.
Observation
Equation
c) a mixture of methanoic acid and ammoniacal silver(I) is heated
Observation
Equation
d) aqueous solution of iodine and sodium hydroxide is warmed with propanone.
Observation
Equation
16. Write equations to show how the following conversions can be carried out.


c) CH3CH2Cl to CH3CONH2
17. a) i) Write the formula and the name of one ore from which aluminum can be extracted.
ii) Name two main impurities in the ore you have named in (b) (i).
b) In the extraction of aluminum, after the removal of the impurities, the ore is mixed with cryolite and then electrolyzed to extract aluminum.
i) State the purpose of adding cryolite.
ii) Name the electrode used in the electrolysis
iii) Write equation for the reaction that takes place at the cathode during the electrolysis.
c) Aluminum powder was added to dilute sodium hydroxide solution.
i) State what was observed.
ii) Write equation for the reaction that took place.
d) Dry chlorine was passed over heated aluminum. Write equation for the reaction that took place.
END.
