1. Which one of the following zinc salts is an insoluble salt?
A. ZnCO3
B. ZnSO4
C. ZnCl2
D. Zn(NO3)2
2. Which one of the following processes increases the amount of nitrogen in the atmosphere?
A. Photosynthesis
B. Haber process
C. Respiration
D. Denitrification
3. A mixture of iron and sulphur was heated. Which one of the following is true about the product?
A. The product is soluble in water
B. The product reacts with acids to produce hydrogen sulphide
C. The product reacts with acids to produce hydrogen
D. Components of the product can be separated using a magnet.
4. The substance formed when little sodium chloride is stirred in plenty of water is called a
A. suspension
B. solvent
C. solution
D. solute
5. Which one of the following processes is used in the conversion of oil into fat?
A. Saponificaiton
B. Dehydration
C. Hydrogenation
D. Polymerisation
6. Which one of the following substances when mixed with water conducts electricity?
A. Kerosene
B. Hydrogen chloride
C. Glucose
D. Carbon tetrachloride
7. Which one of the following alloys can be used for making surgical blades?
A. Brass
B. Bronze
C. Solder
D. Steel
8. Which one of the following statements is true about equal volumes of oxygen and carbon dioxide under the same temperature and pressure? The two gases
A. have equal number of molecules
B. have equal masses
C. have equal density
D. move at the same speed
9. Which one of the following acids, when in a dilute solution will have a pH of about 1?
A. Citric acid
B. Ethanoic acid
C. Carbonic acid
D. Hydrochloric acid
10. The similarity between sulphur dioxide and carbon dioxide is that both
A. are reducing agents
B. turn lime water milky
C. dissolve in water to form acids
D. turn potassium dichromate solution green
11. Which one of the following is used as a catalyst during the laboratory Preparation of oxygen?
A. Iron
B. Platinum
C. Manganese(IV) oxide
D. Vanadium (V) oxide
12. The table below shows the atomic numbers, number of electrons and mass numbers of particles Q,R,X and Y
|
Particle |
Atomic number |
Number of electrons |
Mass number |
|
Q |
19 |
18 |
39 |
|
R |
8 |
8 |
16 |
|
X |
9 |
10 |
18 |
|
Y |
6 |
6 |
12 |
Which one of the particles is a cation?
A. R
B. X
C. Q
D. Y
13. The atomic number of an element Z is 12. What is the atomic number of element W which is immediately below Z in the same group in the Periodic Table?
A. 14
B. 11
C. 13
D. 20
14. Which one of the following gases is produced when iron(III)sulphide is treated with dilute hydrochloric acid?
A. Hydrogen chloride
B. Sulphur dioxide
C. Hydrogen sulphide
D. Chlorine
15. Which one of the following reactions takes place in the absorption tower during the manufacture of nitric acid?
- A. 4NO2(g) + 2H2O(l) + O2(g) à 4HNO3(aq)
- B. NH3(g) + H2O(l) à NH4OH(aq)
- C. 2NO2(g) + H2O(l) à HNO3(aq) + HNO2(aq)
- D. 4NO(g) + 2H2O(l) + 3O2(g) à4HNO3(aq)
16.Which one of the following equations shows formation of hardness in water?
- Ca(HCO3)2(aq) à CaCO3 (s) + CO2(g) + H2O(l)
- CO2(g) + Mg(OH)2(aq) à MgCO3(s) + H2O(g)
- CaCO3(s) à CaO(s) + CO2(g)
- CO2(aq) + MgCO3(s) + H2O(l) à Mg(HCO3)2(aq)
17. The full symbol of the atom is an element X is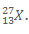 . What is the number of neutrons in the atom of X?
. What is the number of neutrons in the atom of X?
A. 13
B. 14
C. 27
D. 40
18. Which one of the following reactions,that occurs during the manufacture of sulphuric acid by the contact process requires a catalyst?
- à H2S2O7(l)
- à 2H2SO4(aq)
- à 2SO3(g)
- à SO2(g)
19. Which one of the compounds contains the highest percentage by mass of nitrogen?
A.NH4NO3
B.(NH4)2CO3
C. (NH4)3PO4
D. (NH4)2SO4
20. Which one of the following nitrates when heated will decompose to form oxygen as the only gaseous product?
A. AgNO3
B. Zn(NO3)2
C. Ca(NO3)2
D. KNO3
21. A solution of hydrogen chloride in methylbenzene has no effect on litmus paper. This is because hydrogen chloride
A. forms a monobasic acid
B. does not form ions in methylbenzene
C. dissolves to form a dilute acid solution
D. is immiscible with methylbenzene
22. The full symbol of atoms of elements X, Y and Z are respectively. Which one of the following pairs will combine to form a substance with ionic bond?
A. Y and Y
B. X and Z
C. Y and Z
D. X and Y
23. Element Y liberates hydrogen from cold water, whereas W does not W liberates hydrogen from dilute hydrochloric acid, whereas X does not. Which one of the following is the correct order of the reactivity of the elements hydrogen, W, X and Y, starting with the most reactive?
A. Hydrogen, W, X, Y
B. W,X, hydrogen, Y
C. X, hydrogen, Y,W
D. Y, W, Hydrogen, X
24. Ammonia gas reacts with oxygen according to the following equation:
4NH3(g) + 3O2(g) à 2N2(g) + 6H2O(l)
The volume of nitrogen gas formed when 60cm3 of ammonia gas reacts completely with excess oxygen is
A. 20cm3
B.30 cm3
C.120 cm3
D. 240 cm3
25. Which one of the following pairs of substances is used during laboratory preparation of carbon dioxide?
A. Lead(II) carbonate and dilute hydrochloric acid
B. Lead(II)carbonate and dilute sulphuric acid
C. Calcium carbonate and dilute hydrochloric acid
D. Calcium carbonate and dilute sulphuric acid
26. Which one of the following substances is produced at the anode when copper(II) sulphate solution is electrolysed using graphite electrodes?
A. Copper(II) ions
B. Hydrogen
C. Copper
D. Oxygen
27. A hydrocarbon burns in oxygen completely according to the following equation:
C3H8 + xO2(g) à yCO2(g) + zH2O(l)
Which one of the following are the values of x, y and z respectively?
A. 4, 3 and 4
B. 5, 3 and 4
C. 4, 5 and 3
D. 3, 4 and 5
28. Which one of the following hydrocarbons is formed whena mixture of ethanol and concentrated sulphuric acid is heated?
A. C2H6
B. C4H10
C. C3H8
D. C2H4
29. In which one of the following test tubes would a burning splint continue to burn. The test tube containing water and
A. sodium peroxide
B. sodium sulphite
C. sodium hydroxide
D. sodium oxide
30. Which one of the following would be formed when anhydrous copper(II) carbonate is heated?
A. A black solid
B. A green solid
C. A blue solid
D. A brown solid
31. Which one of the following contains the same number of moles of hydrogen ions as the number of moles of sodium ions in 50cm3 of a 0.2M Na2SO4?
A. Al3+
B. Zn2+
C. Pb2+
D. Cu2+
32. Which one of the following contains the same number of moles of hydrogen ions as the number of moles of hydrogen ions as the number of moles of sodium ions in 50cm3 of a 0.2M Na2SO4?
A. 200 cm3 of a 0.1M HNO3
B. 150 cm3 of a 0.2M H2SO4
C. 100 cm3of a 0.5M HCl
D. 50 cm3 of a 1M H3PO4
33. When a mixture of solid Y and concentrated sulphuric acid was heated, a gas that gave dense white fumes with ammonia was evolved. Which one of the following is the anion in Y?
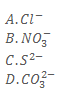
34. Which one of the following salts when reacted with dilute hydrochloric acid can form a white precipitate that dissolves on heating?
A. ZnSO4
B. CuSo4
C. Ba(NO3)2
D. Pb(NO3)2
35.Iron(III) oxide reacts with carbon monoxide according to the following equation:
Fe2O3(s) + 3CO(g) à 2Fe(s) + 3CO2(g)
Which one of the following is the mass of iron obtained when 100g of iron(III) oxide is reduced?
(C = 12; O = 16; Fe = 56)


36. The atomic number of element E is 5. The electronic structure of an element Q which belong to the same group in the Periodic Table is
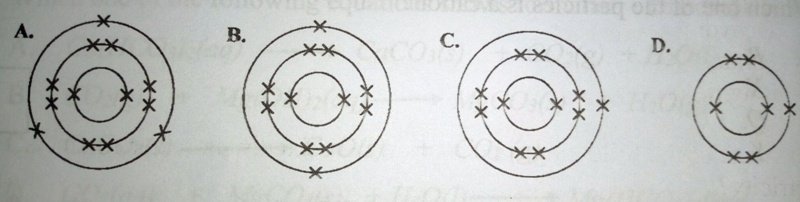
37. Lead(II) nitrate solution reacted with a colourless solution Q to form a yellow precipitate. Which one of the following is the anion in Q?

38. Magnesium carbonate reacts with dilute hydrochloric acid according to the following equation:
MgCO3(s) + 2HCl(aq)à MgCl2(aq) + H2O(l) + CO2(g)
Which one of the following is the mass of magnesium carbonate that would react completely with 100cm3 of a 2M hydrochloric acid?

39. Methanol (CH3OH) burns in air according to the following equation

What would be the amount of heat produced when 20g of methanol is burnt?
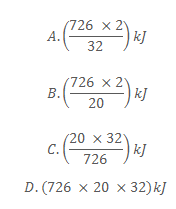
40. When calcium nitrate is strongly heated, it decomposes according to the following equation
2Ca(NO3)2(s) à 2CaO(s) + 4NO2(g) + O2(g)
Which one of the following is the maximum volume of oxygen produced at room temperature when 2.4g of calcium nitrate is heated?
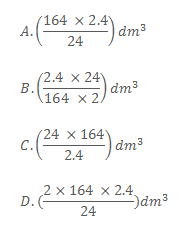
Each of the questions 41 to45 consists of an assertion (statement) on the left hand side a reason on the right –hand side
Select
A. if both the assertion and reason are true statements and the reason is a correct explanation of the assertion
B. if both the assertion and reason are true statements but the reason is a correct explanation of the assertion
C. if the assertion is true but the reason is not a correct statement
D. if the assertion is not correct but the reason is a correct statement
INSTRUCTIONS SUMMARISED
|
Assertion |
Reason |
|
|
A |
True |
True and is a correct explanation |
|
B |
True |
True and is not a correct explanation |
|
C |
True |
Incorrect |
|
D |
Incorrect |
Correct |
41. Diamond and graphite burn in excess oxygen to form carbon dioxide
because they are isotopes of carbon
42. When dry ammonia is passed over heated copper(II) oxide, the oxide changes color from black to brown because copper(II) oxide contain oxygen atoms
43. Elements with atomic number 12 and 17 react to form a covalent compound because the two elements are in the same period in the Periodic Table
44. A white precipitate is formed when solutions of lead(II)nitrate and barium chloride are treated separately with sulphuric acid because metal sulphates do not dissolve in water
45. Chlorine is used in treatment of water because chlorine is an oxidizing agent
In each of the question 46 to 50, one or more of the answers given may be correct. Read each question carefully and then indicate the correct answer according to the following:
A. If 1, 2 and 3 only are correct
B. If 1 and 3 only are correct
C. If 2 and 4 only are correct
D. If 4 only is correct
46. During the extraction of sodium from sodium chloride, calcium chloride is added to fused sodium chlorides so as to
1. make sodium chloride non-corrosive
2. make sodium insoluble in its molten sodium chloride
3.lower the melting poin of sodium chloride
4. remove impurities from the sodium chloride
47. An atom of element X contains 16 electrons and 16 neutrons. Which of the following statements is /are true about X
1. The oxide of X is acidic
2. The atomic number of X is 16
3. X is in period 3 of the Periodic Table
4. X is in group VI of the periodic Table
48. Curves x and y in figure 1 were obtained when a fixed mass of magnesium was reacted separately with a certain volume of dilute sulphuric acid
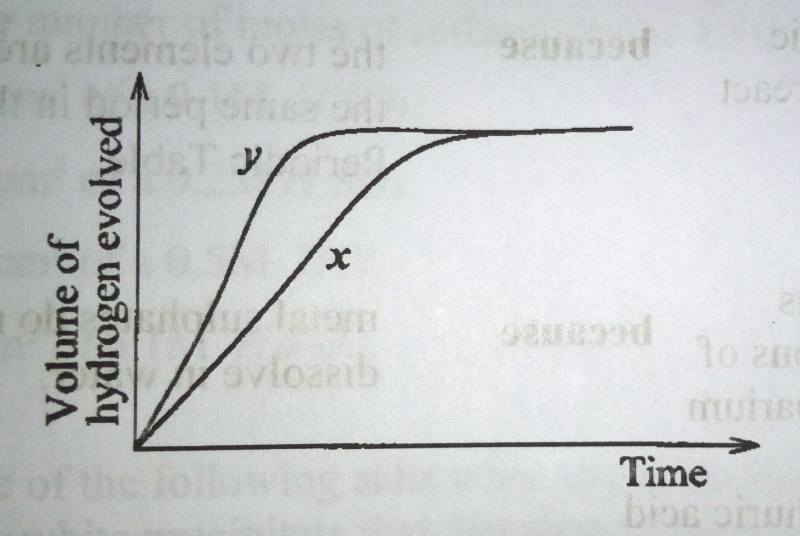
The condition(s) under which y was obtained is/are by
1. using magnesium ribbon
2. increasing the concentration of the acid
3. reducing the reaction temperature
4. using magnesium powder
49. Which of the following compounds decolorizes bromine water
1. CH4
2. C3H8
3. C4H10
4. C2H4
50.Which of the following is/are formed when nitric acid is reacted with a metal oxide?
1. Water
2. Oxygen
3. Nitrate of the metal
4. Nitrogen dioxide gas
END
